Facts About Dye Dilution Revealed
Wiki Article
Dye Dilution Things To Know Before You Buy
Table of ContentsThe Ultimate Guide To Dye DilutionSome Ideas on Dye Dilution You Need To KnowIndicators on Dye Dilution You Need To KnowTop Guidelines Of Dye DilutionWhat Does Dye Dilution Do?About Dye DilutionOur Dye Dilution PDFsLittle Known Facts About Dye Dilution.The Only Guide for Dye Dilution
Serial dilutions are made by making the same dilution action over as well as over, making use of the previous dilution as the input to the next dilution in each step. Because the dilution-fold coincides in each step, the dilutions are a geometric series (constant proportion between any type of surrounding dilutions). For instance: Notice that each dilution is three-fold about the previous one. 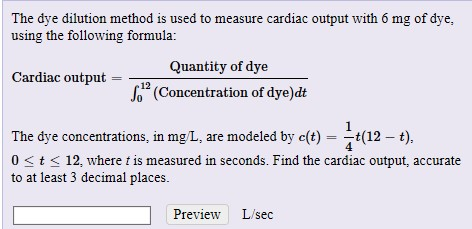
Some Known Details About Dye Dilution
This prevents bunching most of the points up at one end as well as having just the last factor means much down the scale. Prior to making serial dilutions, you need to make harsh price quotes of the concentrations in your unknowns, and also your uncertainty in those price quotes. If A280 says you have 7.7 and also 7 mg/ml. That means you need to cover a ten-fold series of dilutions, or possibly a bit extra to make sure. If the half-max of your assay happens at regarding 0. 5 mg/ml, after that your minimum dilution fold is (700 mg/ml)/(0. 5 mg/ml) = 1,400. Your optimum is (7000 mg/ml)/(0.
About Dye Dilution
So to be secure, you might intend to cover 1,000 via 20,000. In basic, before designing a dilution series, you need to determine: What are the least expensive and also greatest concentrations (or dilutions) you require to check in order to be particular of finding the half-max? These figure out the series of the dilution collection.You need to make a 1/1,000 dilution to begin with. You could make 1/1,000 by including 1 microliter of sample to 0.
What Does Dye Dilution Do?
Why is that a poor choice? Because you can't measure 1 microliter (and even 10 microliters) precisely with average pipeters. So, make 3 serial 1/10 dilutions (0. 1 ml [100 microliters] into 0. 9 ml): 1/10 x 1/10 x 1/10 = 1/1,000. Now you might add 1. 0 ml of the beginning 1/1,000 dilution to 1.Remove 1. 0 ml from that dilution (leaving 1. 0 ml for your tests), and add it to 1. 0 ml of diluent in the next tube (offering 1/4,000). Etc for 3 more serial dilution actions (offering 1/8,000, 1/16,000, and also 1/32,000). You wind up with 1 (Dye Dilution). 0 ml of each dilution.
8 Easy Facts About Dye Dilution Shown
Water is the most abundant element in the human body consisting of regarding 60% of body mass in the reference man. Because it is mostly located in the fat-free body in a reasonably continuous quantity, analysis of body water has actually been of interest as a technique of body composition analysis for practically 100 years.Water's characteristic as a particular molecular varieties uses itself to making use of the dilution concept, which in its easiest type, mentions that the quantity of the component amounts to the quantity of isotope included in the part separated by the concentration of the isotope because component. In 1915, the dilution principle was initially used in the study of body composition when making use of a red dye to page measure the plasma quantity was theorized.
Fascination About Dye Dilution
Utilizing a mathematical strategy, a practical price quote was made to calculate the quantity of plasma in which the color was very first weakened. Following this examination and making use of the very same concept, tracer material was injected intravenously as well as permitted to reach a consistent distribution, and from the dilution accomplished at equilibrium, the constituents of the body were measured.Tritiated water was initial explained by Pace et al. as an isotope for measuring TBW - Dye Dilution. The main benefit of making use of tritium (3H), the radioactive isotope of hydrogen, is that it is readily available as well as conveniently assayed by scintillation counting. On the other hand, a big amount of tritiated water have to be administered to obtain appropriate accuracy, eliminating its usage in cases where using radionuclides is restricted.
The 6-Second Trick For Dye Dilution
Greater technological errors have been found making use of the infrared method. When using isotope dilution, especially deuterated water, 2 body fluid examples from pee, blood, or saliva are collected: one prior to administration of the deuterium dose to determine the natural history degrees and also the 2nd after permitting sufficient time for penetration of the isotope.There are four fundamental presumptions that are fundamental in any isotope dilution technique. Tracer exchanges with nonaqueous molecules are marginal, and also subsequently, the volume of circulation or dilution area of the isotope can be determined, albeit somewhat higher than the water swimming pool.
Our Dye Dilution Diaries

Still, it is necessary to consider voids after tracer management. Three voids are suggested after the dose when urine is utilized as the biological example. The tracer is not metabolized throughout the equilibration time. Body water remains in a continuous state of flux. In warm environments, the average fractional turn over rate in i was reading this grownups is 8% to 10% every day.
Fascination About Dye Dilution
The inputs are stabilized by an outcome of water in the kind of urine, sweat, breath water, or transdermal dissipation. This continuous turnover has actually brought about two methods when assessing TBW: the plateau technique and the back-extrapolation, or slope-intercept, technique. For body composition research, the plateau method is the normal technique.Report this wiki page